Light is Vital for Photosynthesis
Generally speaking, there are cells within the ‘eye’ that exist only to capture light, hence the human term, ‘visible light spectrum’. Plants are multicellular autotrophs with cell walls made of cellulose. Autotrophs make their own food and accomplish this through the photosynthesis.
Light spectrum provides plants with information about the environment for germination, to grow certain shapes, sizes or colours, and to protect themselves, (with angiosperms) when they flower or fruit. Plant species vary in behaviour and are not all that different from one another but generally they all proceed to grow and form from the light source’s quality, intensity, duration and direction.
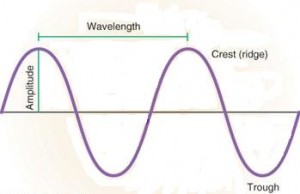
Light is vital for photosynthesis, and it is necessary to direct plants growth and development. Light acts as a signal to initiate and regulate photoperiodism and photomorphogenesis. Light waves have crests and valleys of a particular height or amplitude. This parameter of waves is perceived as brightness or intensity.
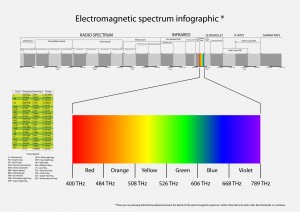
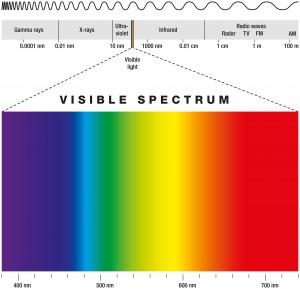
The general areas which plants receive optimal levels of light are in the ranges from 400–700 nm which is called Photosynthetically Active Radiation (PAR). PAR is a measurement of quantum units, when considered as a wave; light has a wavelength and a frequency.
Light as a Wavelength
Highest Energy ⇔ Lowest Energy
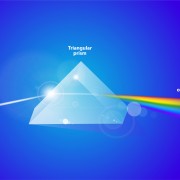
Spectroscopy or spectrum pertains to the dispersion of an object’s light into its component colors (i.e. energies). There are a range of units for this parameter, both English and Metric depending on the source, the surface, or the passage. Light can also be thought of as a stream of particles, photons, or quanta. In this case, units can be expressed in moles per square meter per second (mol m–2 s–1), where “moles” refers to the number of photons (1 mol of light = 6.02 × 1023 photons, Avogadro’s number). This measure is called photon. It is used commonly to describe PAR in the range of 400-700 nanometres (nm) wavebands.
Light as a Frequency
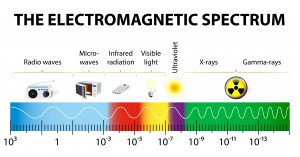
Electromagnetic radiation spans an enormous range of frequencies or wavelengths, as is shown by the electromagnetic spectrum. Customarily, it is designated by fields, waves, and particles in increasing magnitude of frequencies–radio waves, microwaves, infrared rays, visible light, ultraviolet light, X rays, and gamma rays. The corresponding wavelengths are inversely proportional, and both the frequency and wavelength scales are logarithmic which is a nonlinear scale used when there is a large range of quantities.
The common uses include the earthquake strength, sound loudness, light intensity, and potential hydrogen (pH) of solutions.
The Electromagnetic Spectrum
The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The “electromagnetic spectrum” of an object has a different meaning, and is instead the characteristic distribution of electromagnetic radiation emitted or absorbed by that particular object.
The electromagnetic spectrum extends from below the low frequencies used for modern radio communication to gamma radiation at the short-wavelength (high-frequency) end, thereby covering wavelengths from thousands of kilometers down to a fraction of the size of an atom.