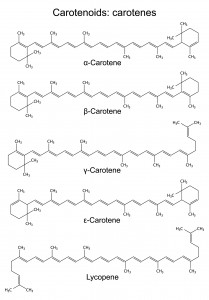
Chlorophyll
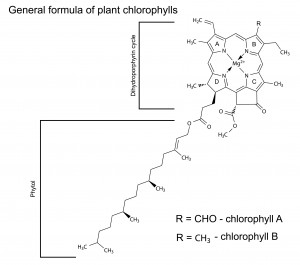
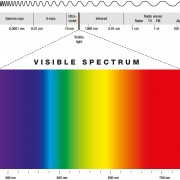
Chlorophyll is the primary photosynthetic pigment class and its structure reveals the mechanism of its absorption of light, the excitation of its electrons, and its ability to give electrons off to an electron transfer system. Chlorophyll absorbs photons at wavelengths of between 400 and 700nm and channels their energy into the process of photosynthesis. The molecules of chlorophyll (C55H70MgN4O6) are large. Instead, they are attached to the membranes of disc-like structures, called chloroplasts, inside the cells.
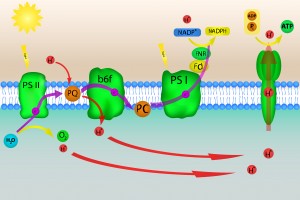
Photosynthesis occuring in chloroplast of plants
Chloroplasts are the site of photosynthesis, the process in which light energy is converted to chemical energy. The photons travelling along the waves enter the thylakoid space within chloroplasts. The light absorbed by chlorophyll space (which lines the inside of the thylakoid) supplies the energy used by plants to transform carbon dioxide and water into oxygen and carbohydrates. It will have a general formula of Cx(H2O)y. Basically, the chlorophyll vibrates because of the energy from the trapped photon. It can then transfer the photon to be used as energy.
| x CO2 + y H2O | light | x O2 + Cx(H2O)y |
| chlorophyll |
This is a chemical change that is accompanied by absorption of heat (endothermic transformation). The energy of the light absorbed by chlorophyll is converted into chemical energy stored in carbohydrates (sugars and starches). This chemical energy drives the biochemical reactions that cause plants to grow, flower, and produce seed.
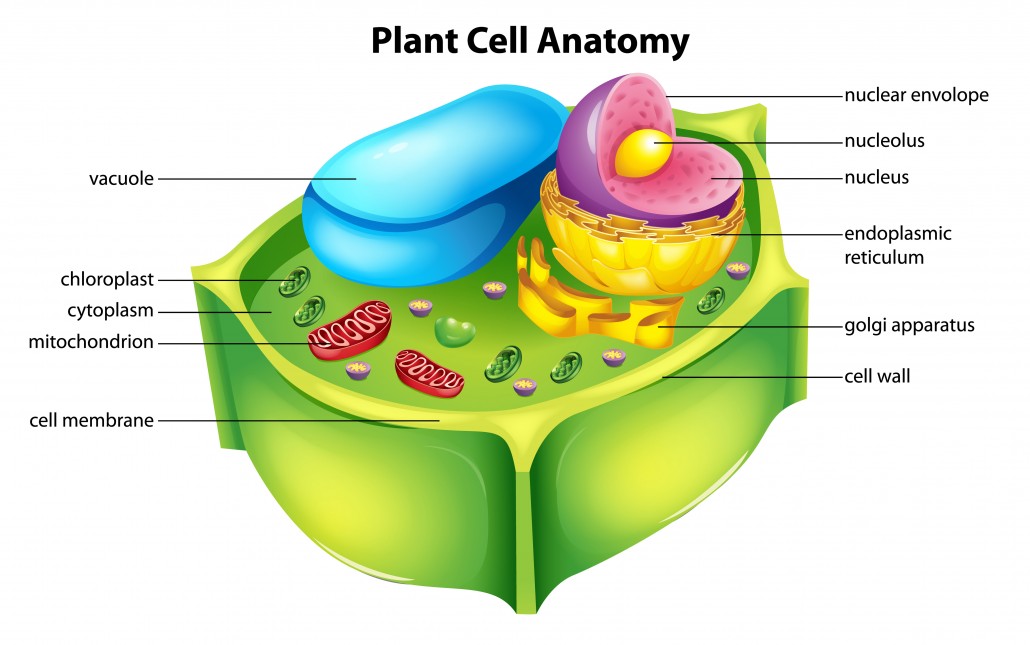
Plant cell Anatomy
Plants can sense and respond to different stimuli such as chemical concentrations in the air and soil, to water, from being touched, any motion or vibration that may be present in the environment around them, pathogens, predators and also light and spectra heavily influence their development.
“Recent biochemical and molecular work is producing a growing list of elements involved in responses to biotic and abiotic stimuli that are very similar across kingdoms. Some of the more interesting examples of these include prostaglandin/octadecanoid-mediated responses to wounding, steroid-based signaling systems, and pathogen-recognition mechanisms. Some of these similarities probably represent evolutionary convergence; others may be ancestral to plants and animals. Ecological and evolutionary implications of such overlaps include the existence of pathogens that can cause disease in plants and animals, the ability of herbivores to manipulate plant responses, usurpation of microbial mechanisms and genes by herbivorous animals and plants, evolution of plant defenses exploiting shared signals in animals, and the medicinal use of plants by humans. Comparative study of the signaling and response mechanisms used by plants, animals, and microbes provides novel and useful insights to the ecology and evolution of interactions across kingdoms.”
When working with LEDs to grow and cultivate the wide variety if plant life that we consume one begins to realize that there are vast variables which we are just beginning to understand when it comes to the relationship between plants and animals. It has been suggested that plants and animals share the same molecular pathways in order to respond to stress, it is convincing to say the least that a molecule produced in plants can also be effective in animals. Plants create a wide array of protective substances some of which can induce similarly protective responses in those who eat them.